Fda Cder Org Chart - Food And Drug Administration An Overview Sciencedirect

Food And Drug Administration An Overview Sciencedirect
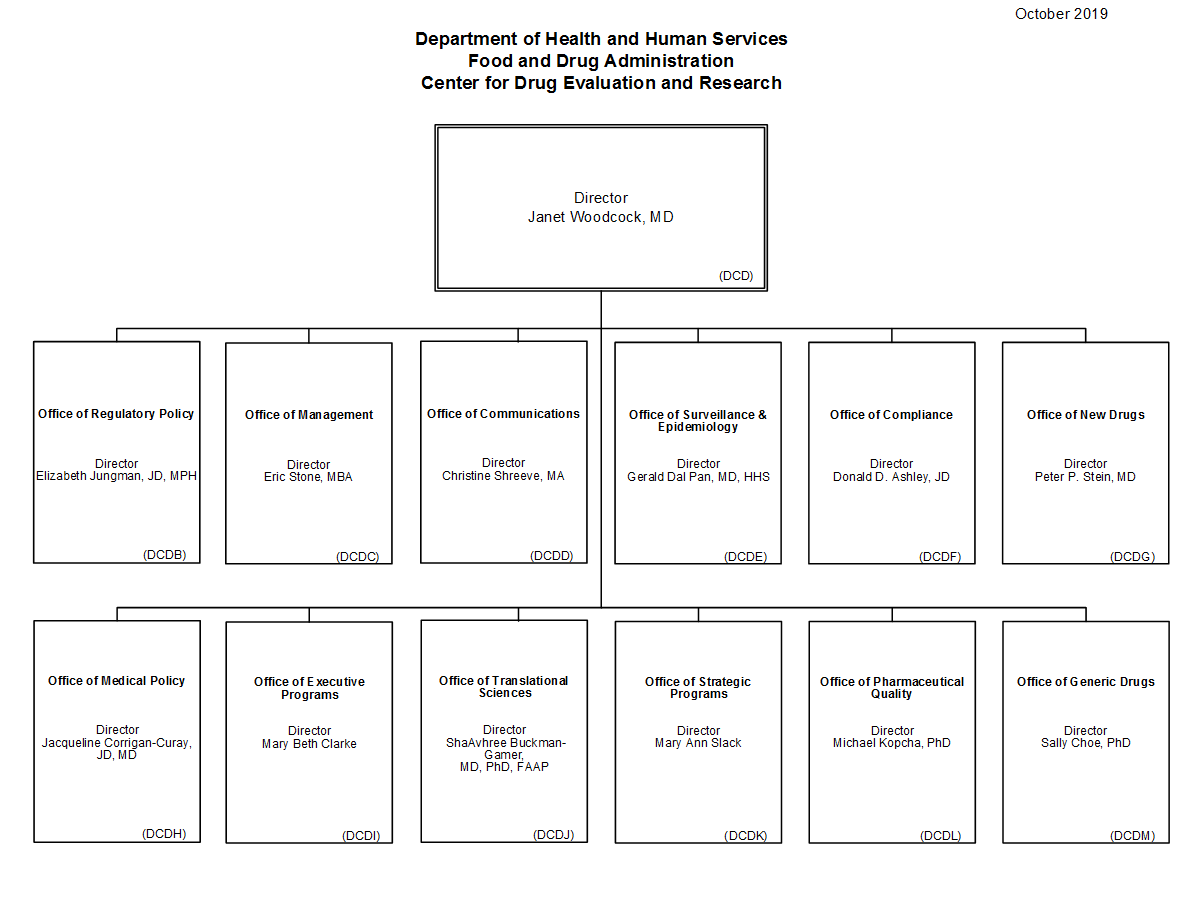
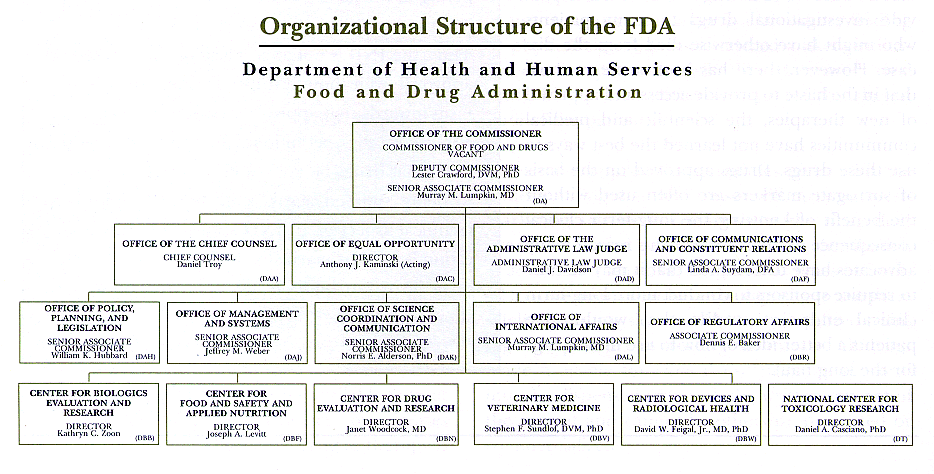
Center For Drug Evaluation And Research Organization Chart Fda .
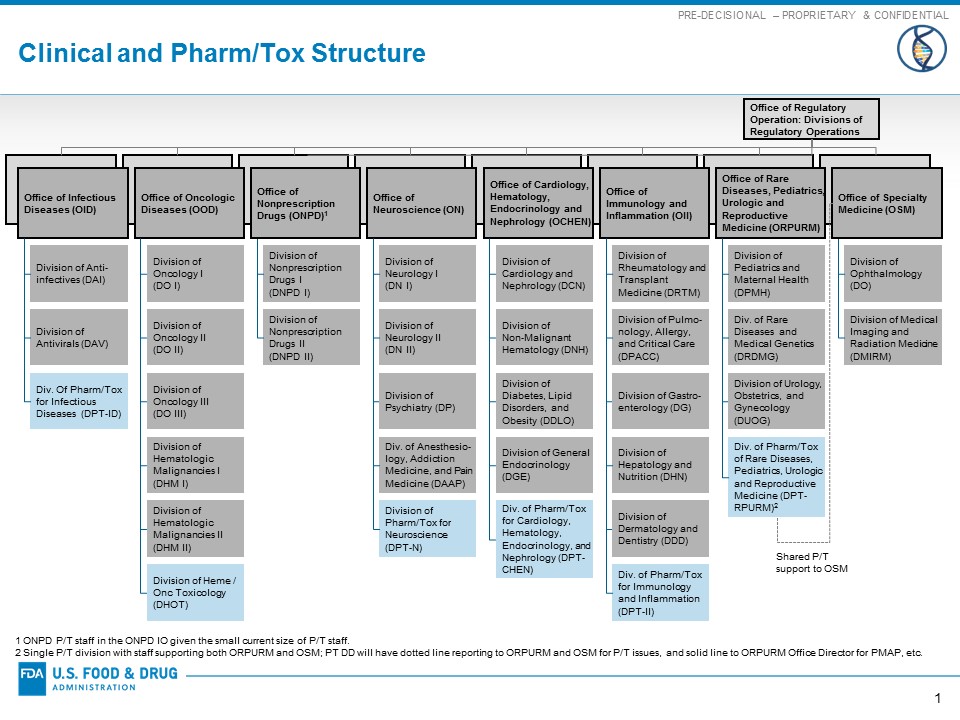
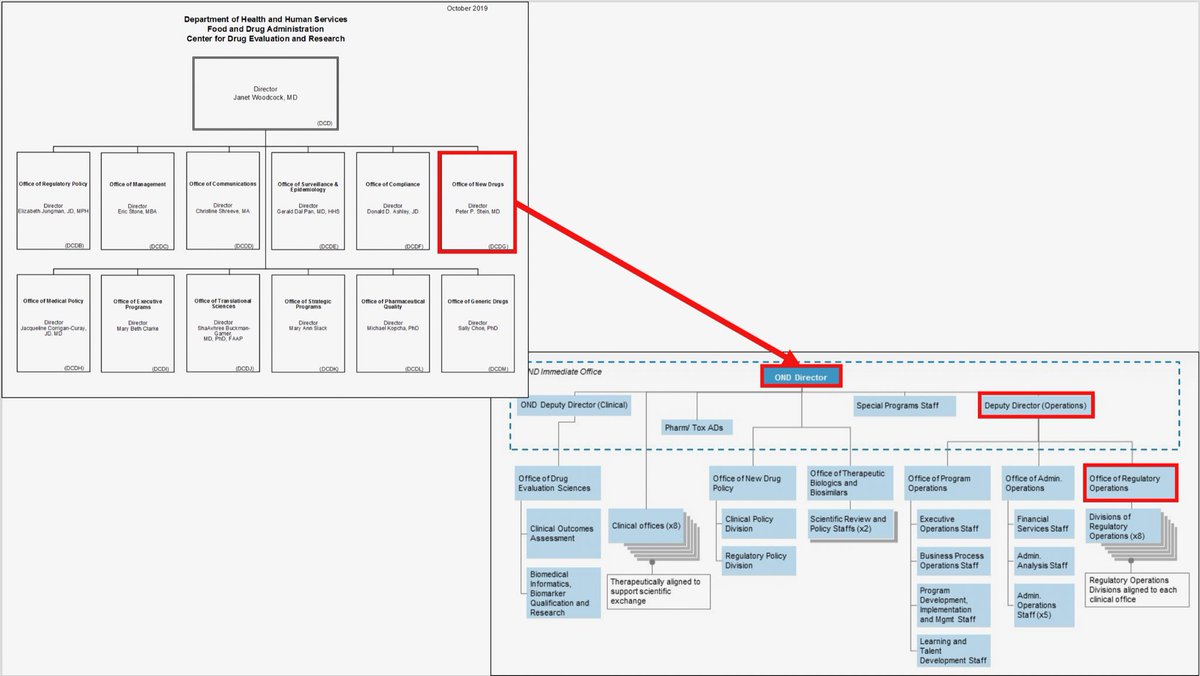
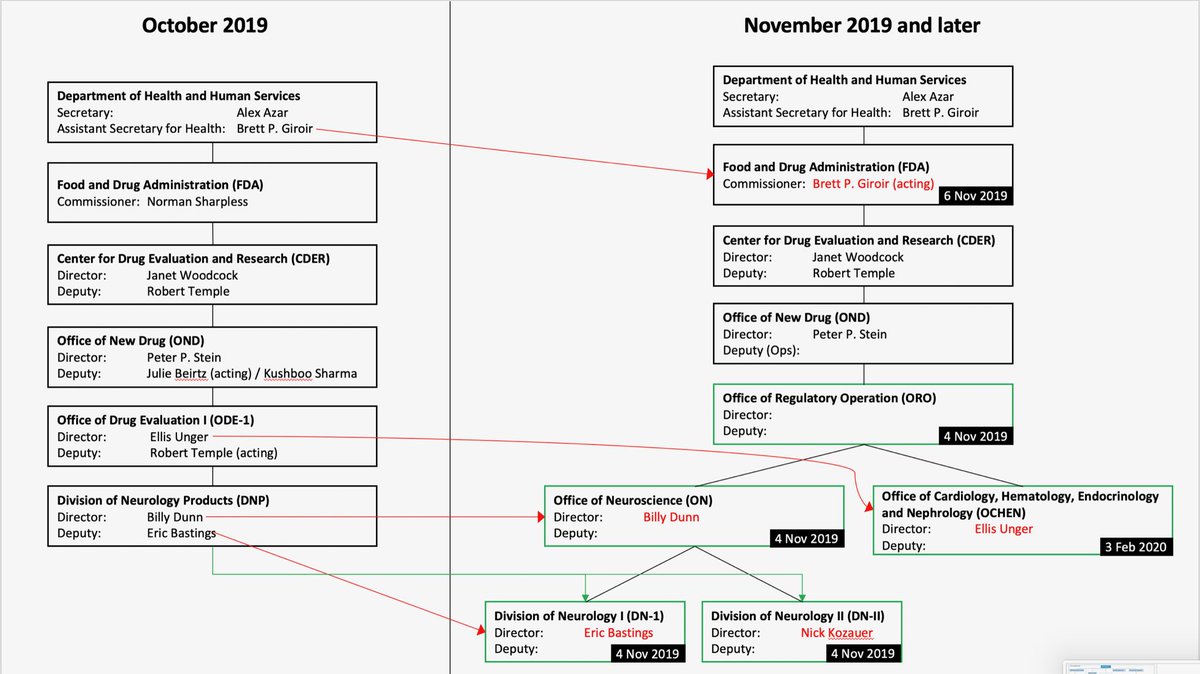
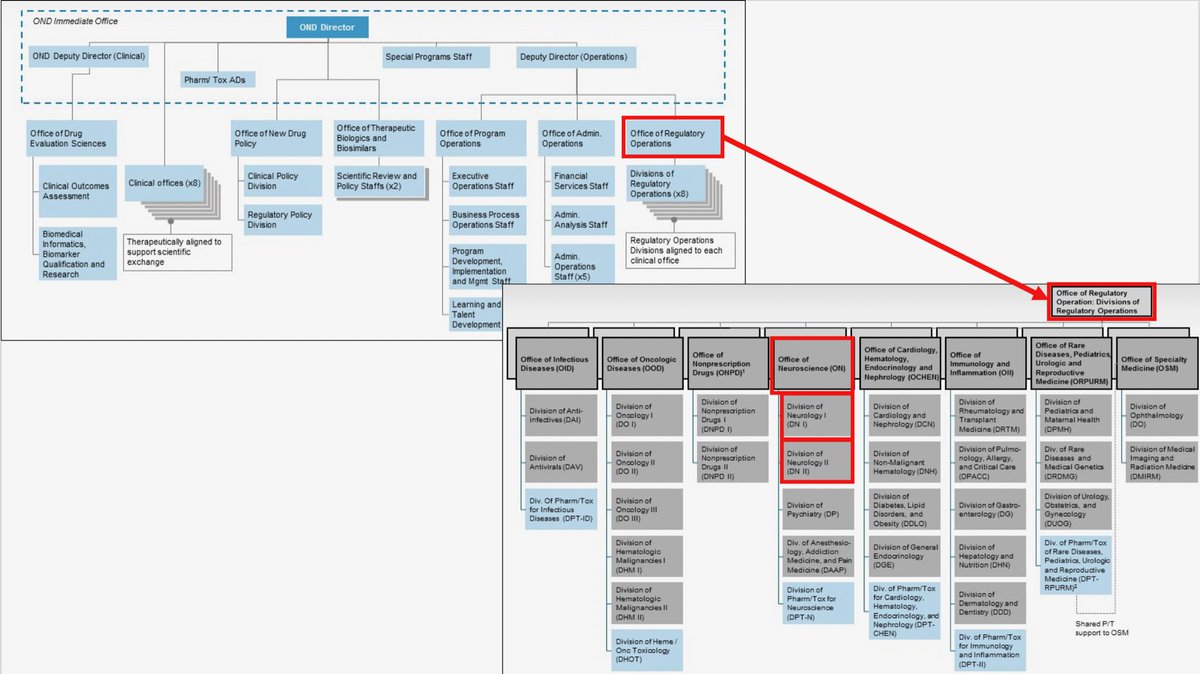
Reorganization Of The Office Of New Drugs With Corresponding .
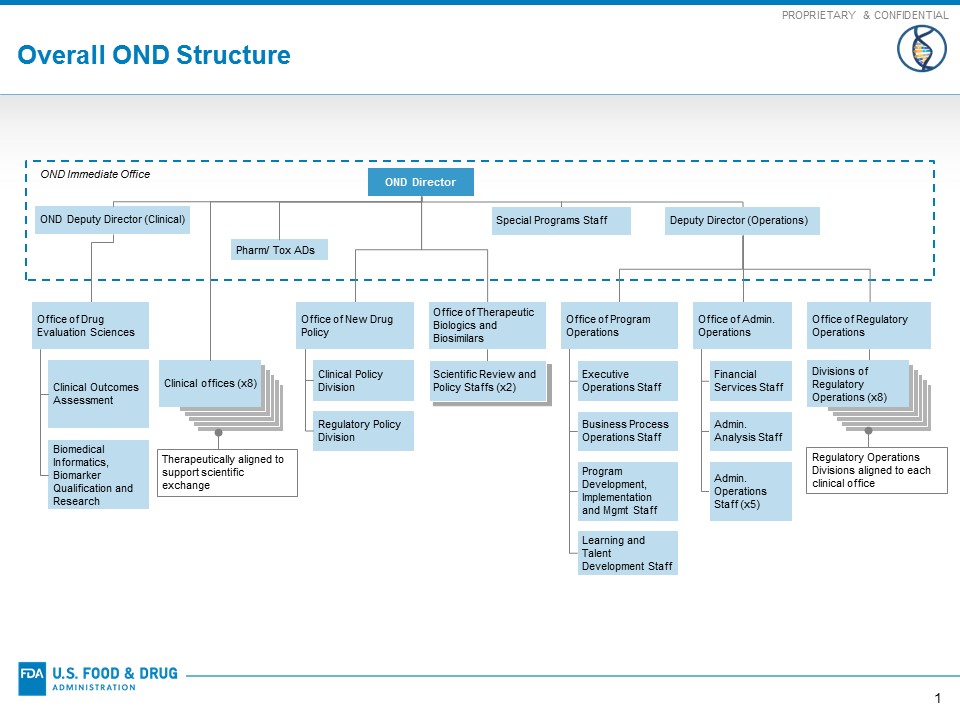
Reorganization Of The Office Of New Drugs With Corresponding .

Center For Drug Evaluation And Research Cder Tanya Eberle .

Office Of Biostatistics Center Of Drug Evaluation And .

1 Office Of Biostatistics Center Of Drug Evaluation And .
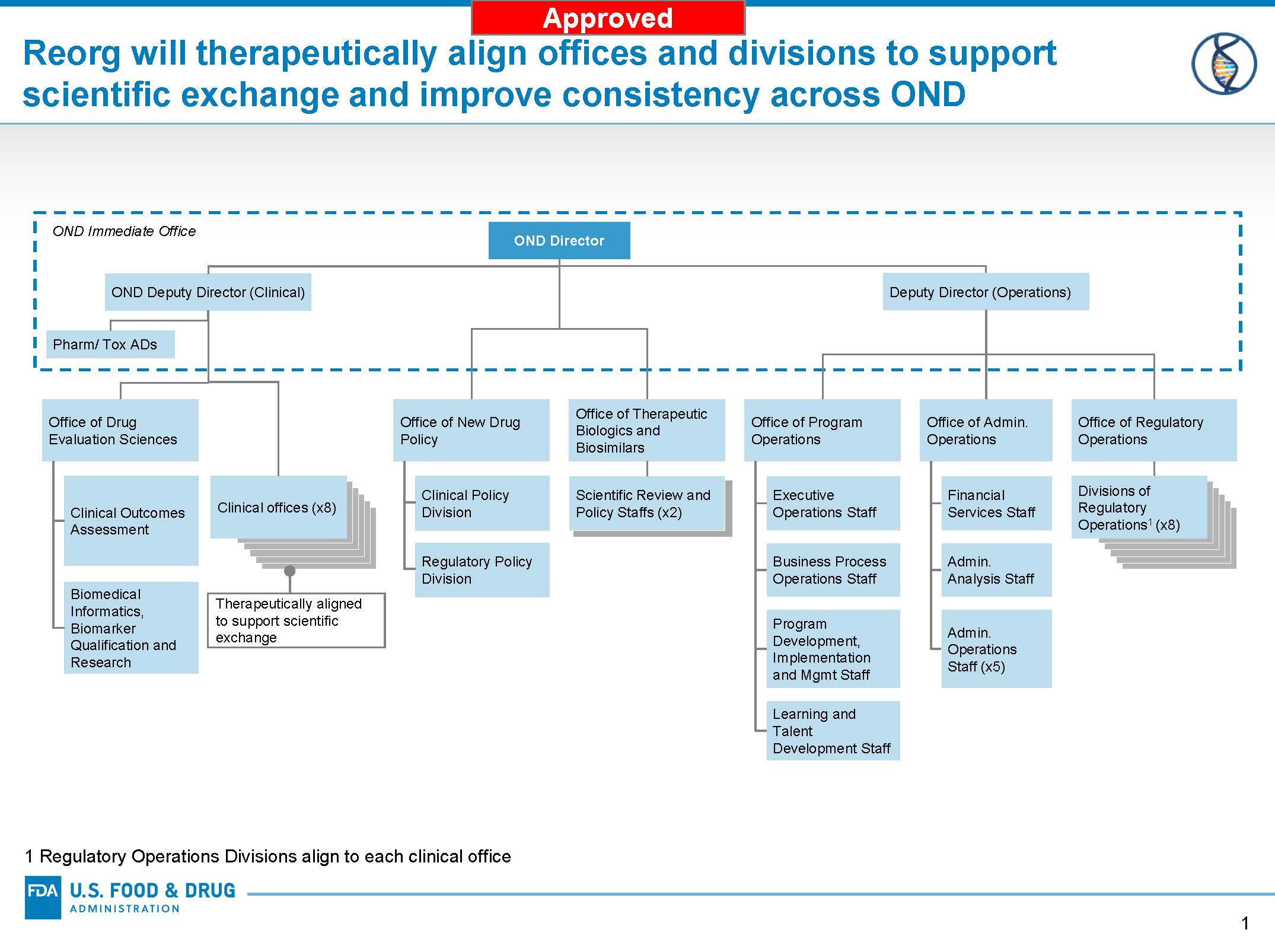
Reorganization Of The Office Of New Drugs With Corresponding .

Cder Compliance Super Office Structure Supply Chain Threats .
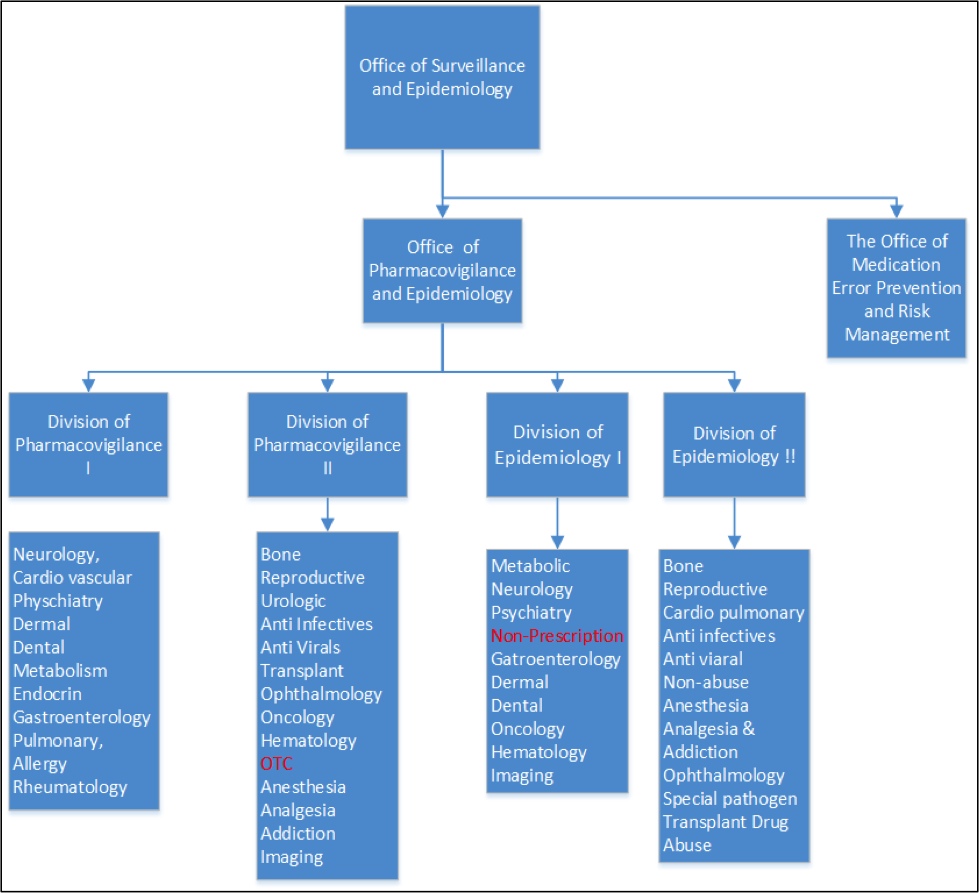
Fda Office Of Surveillance And Epidemiology The American .

21 Eye Catching Fda Cber Org Chart .

Organizational Chart .

Proposed Cder Reorganization Washington Dc Nov 4 .

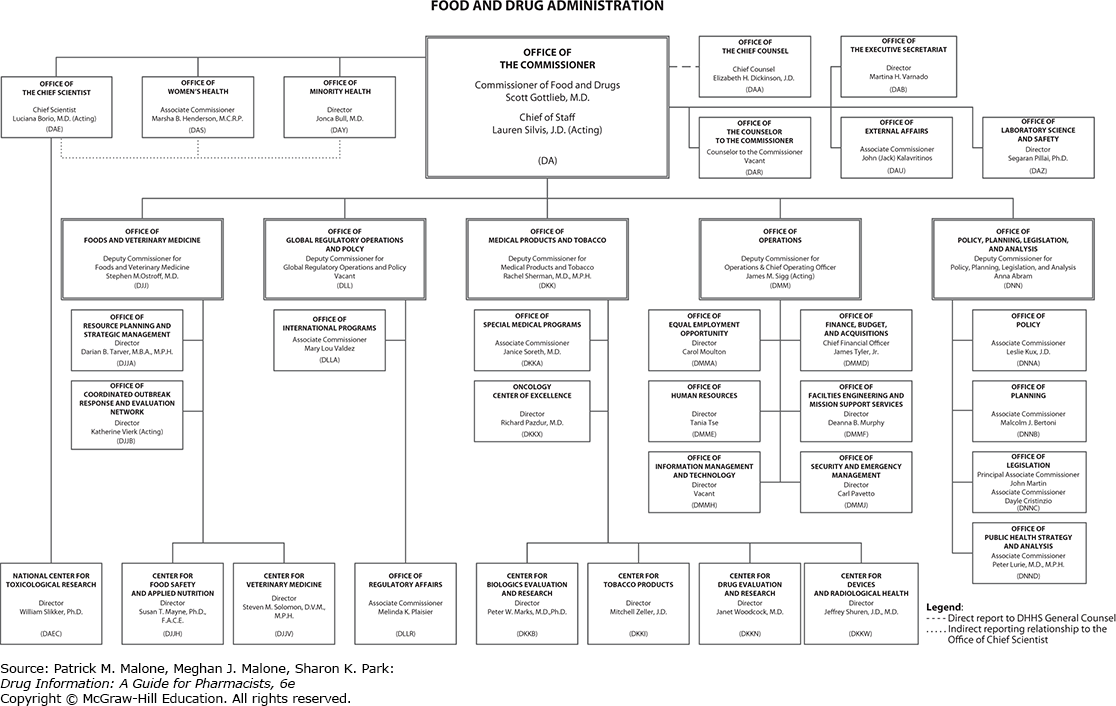
Food And Drug Administration An Overview Sciencedirect .
An Overview Of The Drug Approval Process Thebodypro .
Pharmaceutical Industry And Regulatory Affairs Drug .

21 Eye Catching Fda Cber Org Chart .
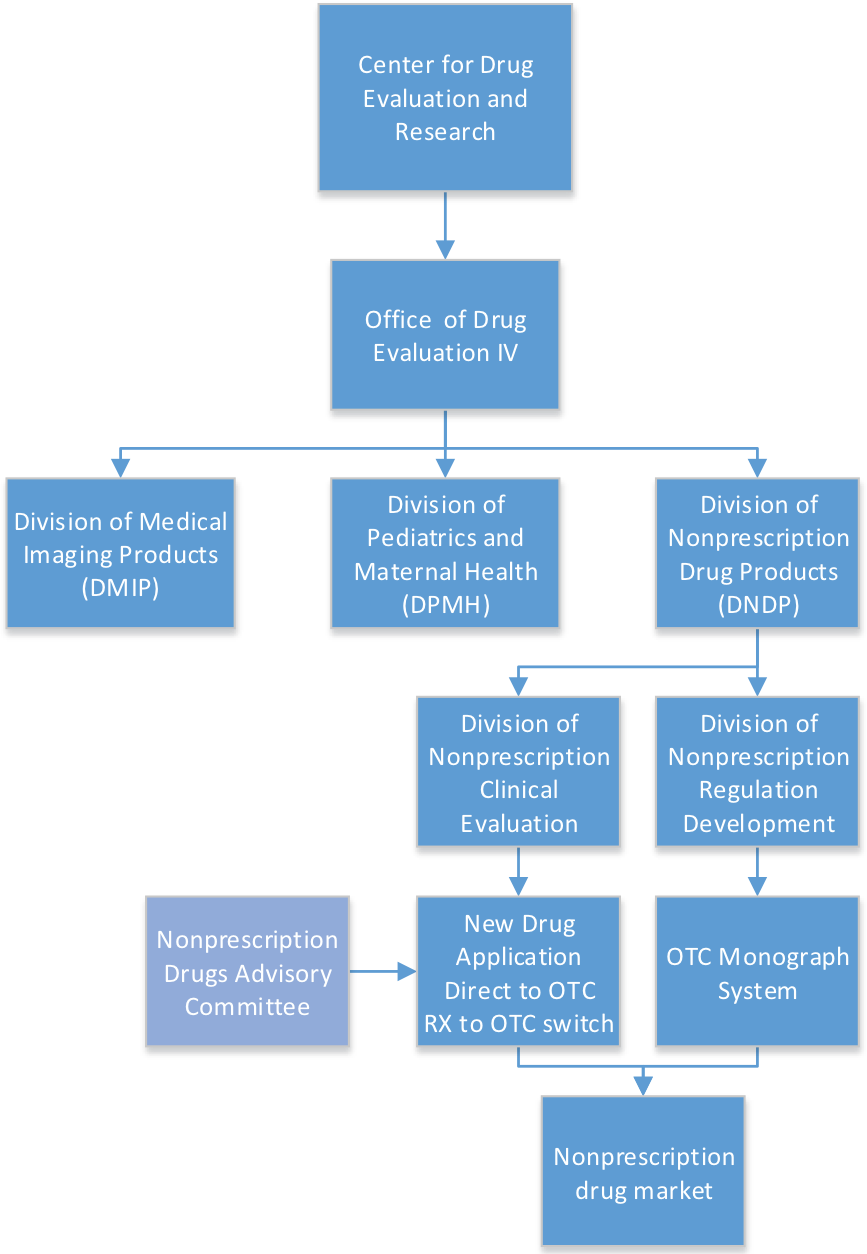
Fdas Division Of Nonprescription Drug Products The .

Center For Drug Evaluation And Research Cder .

The Us Food And Drug Administrations Center For Drug .

Title Page Quality Mo Samimi Ph D Cmq Oe Presented At The .

Working With The Fda Life Cycle Of Product Lifesciences Bc .

Figure 1 From A Program To Provide Regulatory Support For .

Meetings With Cder Judit Milstein Pdf Free Download .
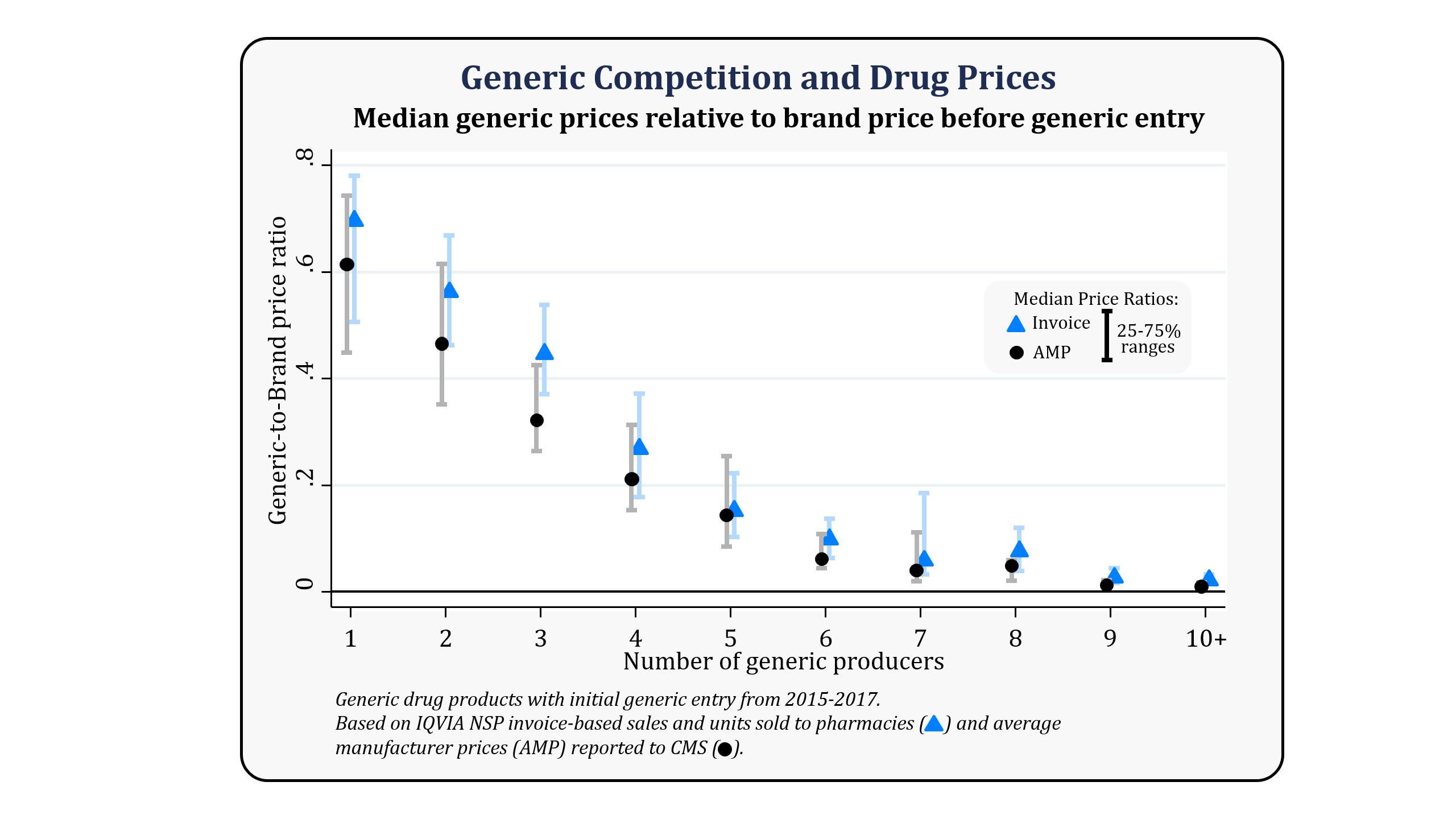
Generic Competition And Drug Prices Fda .

Ascrs Advocacy Prompts Fda To Propose Separate Drug Division .

Sidelining Safety The Fdas Inadequate Response To The Iom .

Fda Pai Compliance Program Guidance Cpg 7346 832 Ipq .

Figure 2 From Clinical Trials In Orthopaedics Research Part .
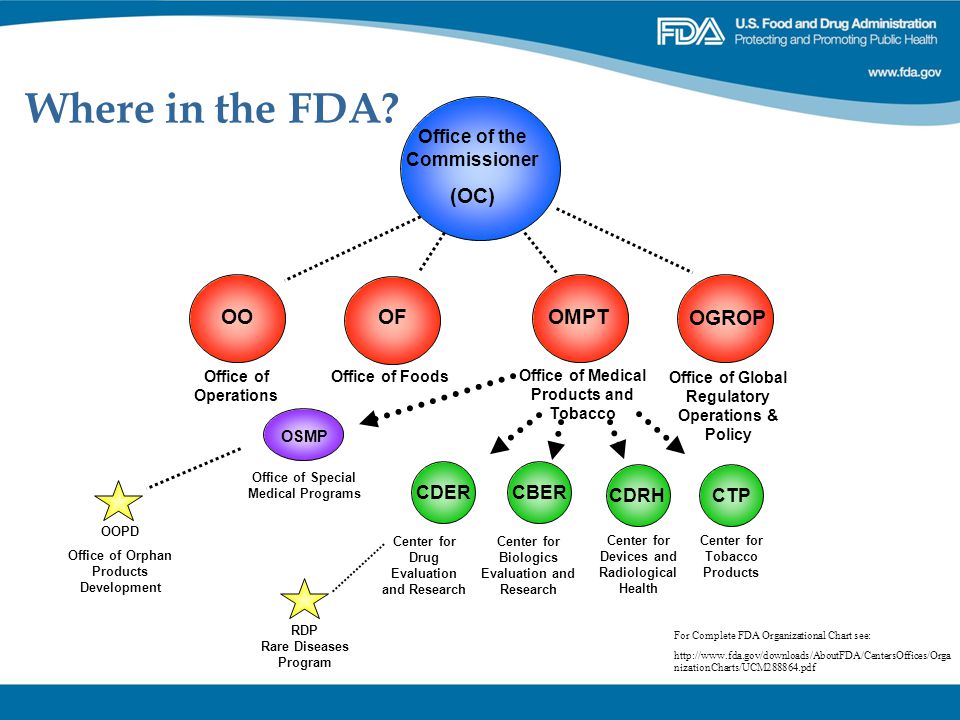
Rare Diseases And Fdasia Ppt Video Online Download .
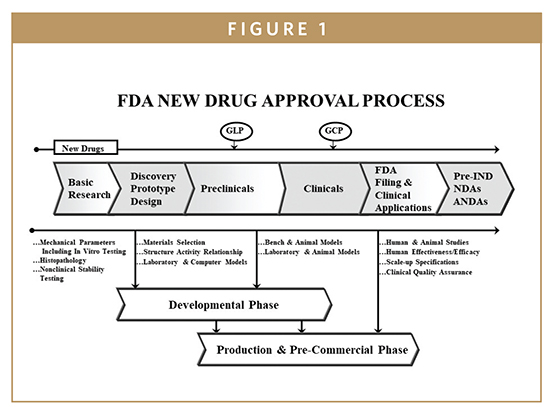
Fda Update The Fdas New Drug Approval Process .

Summary Of Fda Regulations On Exemption From Ind Research .

Fda Module 1 Grouped Submissions Q A The Ectd Summit .
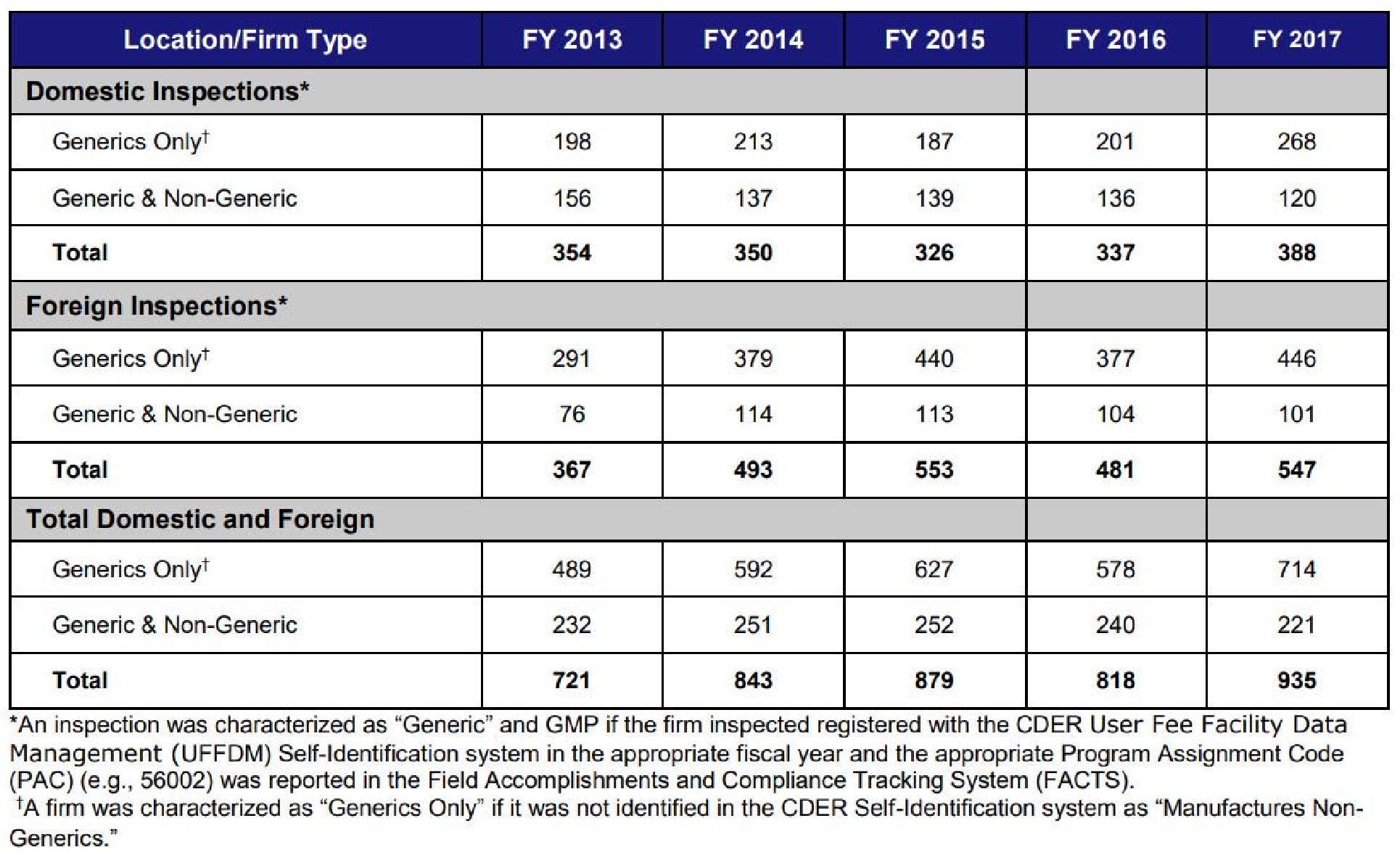
Generic Prescription Drugs Your Health Our Commitment .

Summary Level Information And Data For Cder S Inspection .

Office Of Biostatistics Center Of Drug Evaluation And .

Meetings With Cder Judit Milstein Pdf Free Download .

Product Registration And Drug Approval Process In The United .

Data Driven How Oncologists Perceive Fda Approved Drug .

Novel Fda Drug Approvals For 2017 Sharevault .

Center For Drug Evaluation And Research Cder .

Novel Fda Drug Approvals For 2017 Sharevault .

Summary Of Fda Regulations On Exemption From Ind Research .

Summary Level Information And Data For Cder S Inspection .

Drug Product Approval In The United States And International .
Pharmaceutical Industry And Regulatory Affairs Drug .
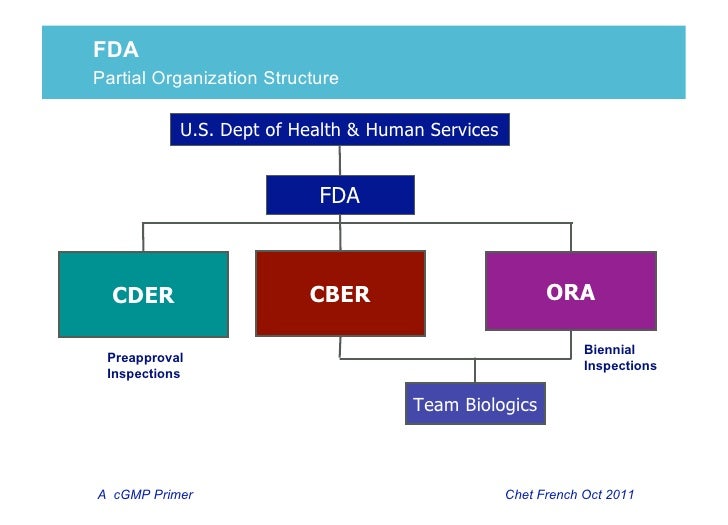
Fda Cder Organizational Chart 2018 Related Keywords .
- ppl center seating chart phantoms
- eagle claw split shot size chart
- quick pie chart generator
- yahoo finance dow jones chart
- treasury chart
- charleston wv civic center seating chart
- metal chart holder
- medical grade ring binder charts
- lamb meat chart
- hockey elbow pad size chart
- ralph robe size chart
- zutano booties size chart
- mint velvet size chart
- pac amp seating chart
- medela bra size chart
- criminal law defenses chart
- greek alphabet chart printable
- clavamox for dogs dosage chart
- barco one size chart
- spa filter cross reference chart
- yuasa battery size chart
- radio city music seating chart
- camisole size chart
- merrick puppy food chart
- dancing queen dress size chart
- homemade reward charts for toddlers
- sakrete color chart
- exhaust pipe id od chart
- chef coat size chart
- wedding cake chart