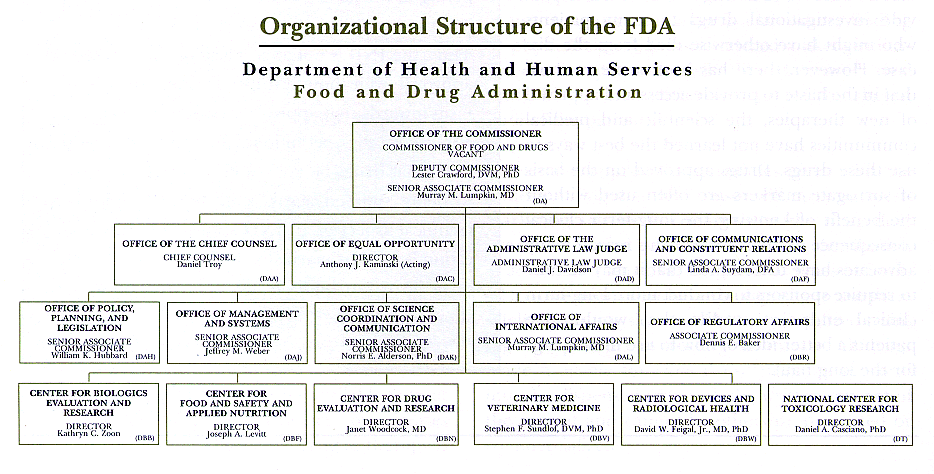
Fda Cder Org Chart - Center For Drug Evaluation And Research Organization Chart Fda
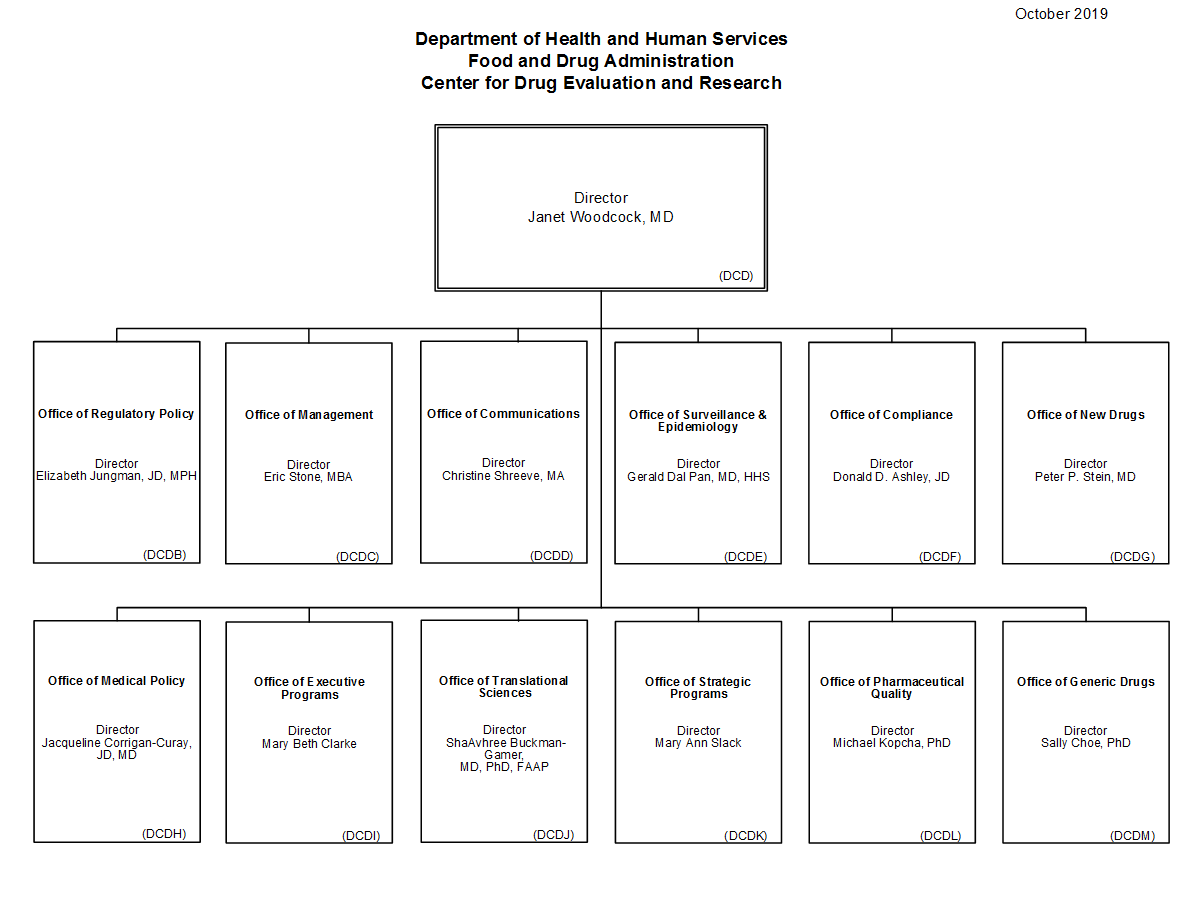
Center For Drug Evaluation And Research Organization Chart Fda .
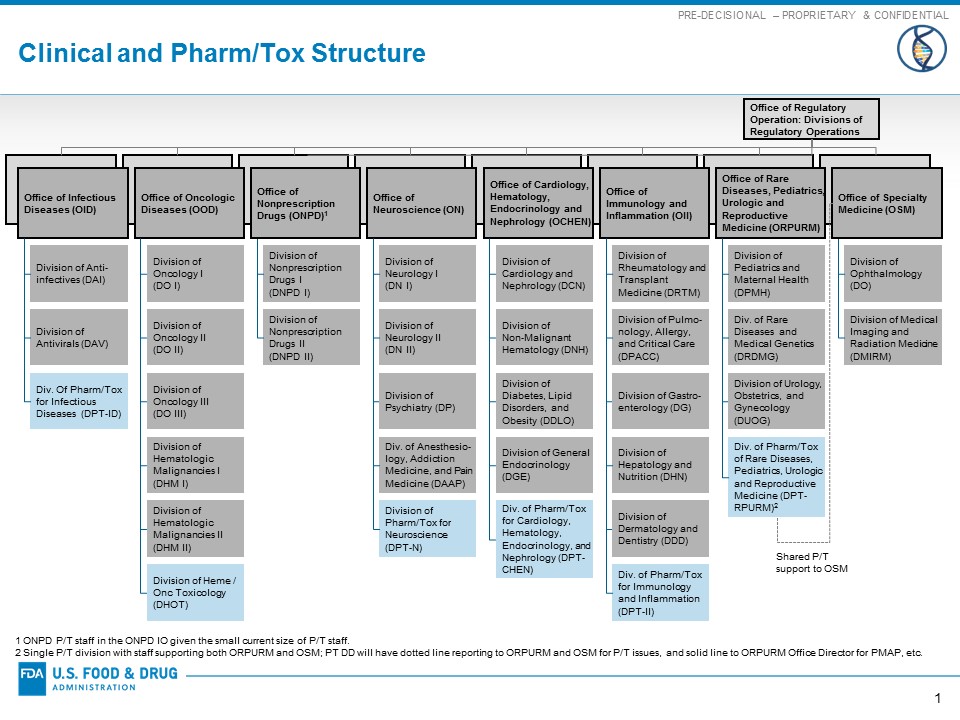
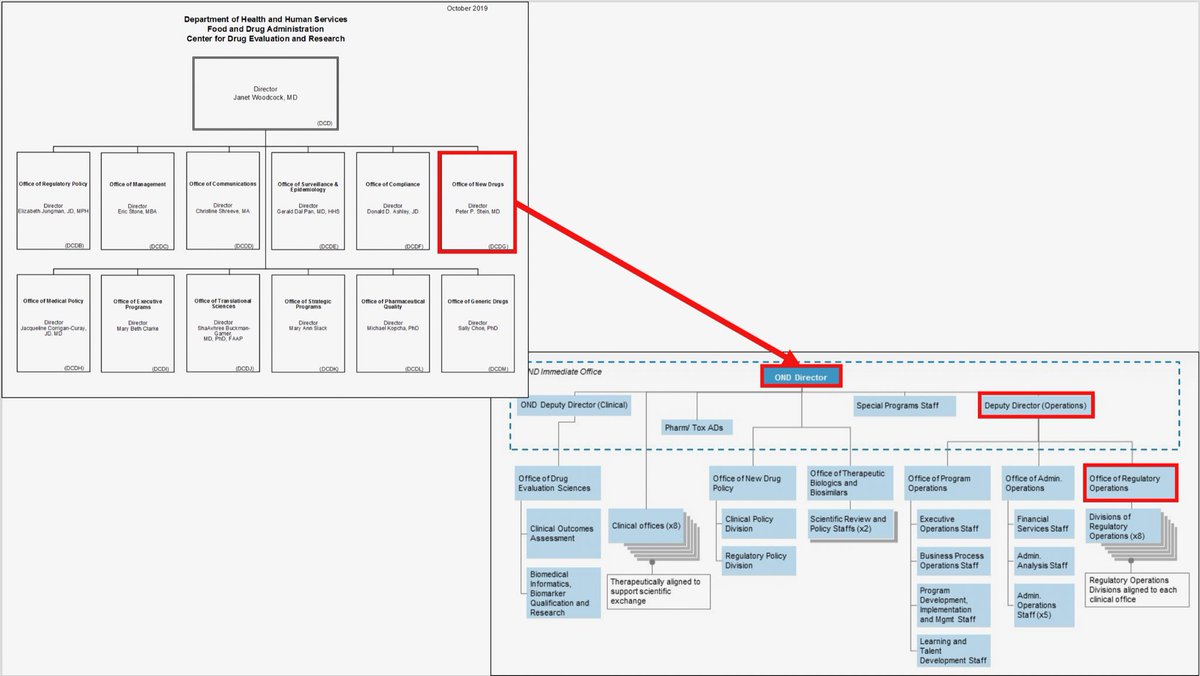
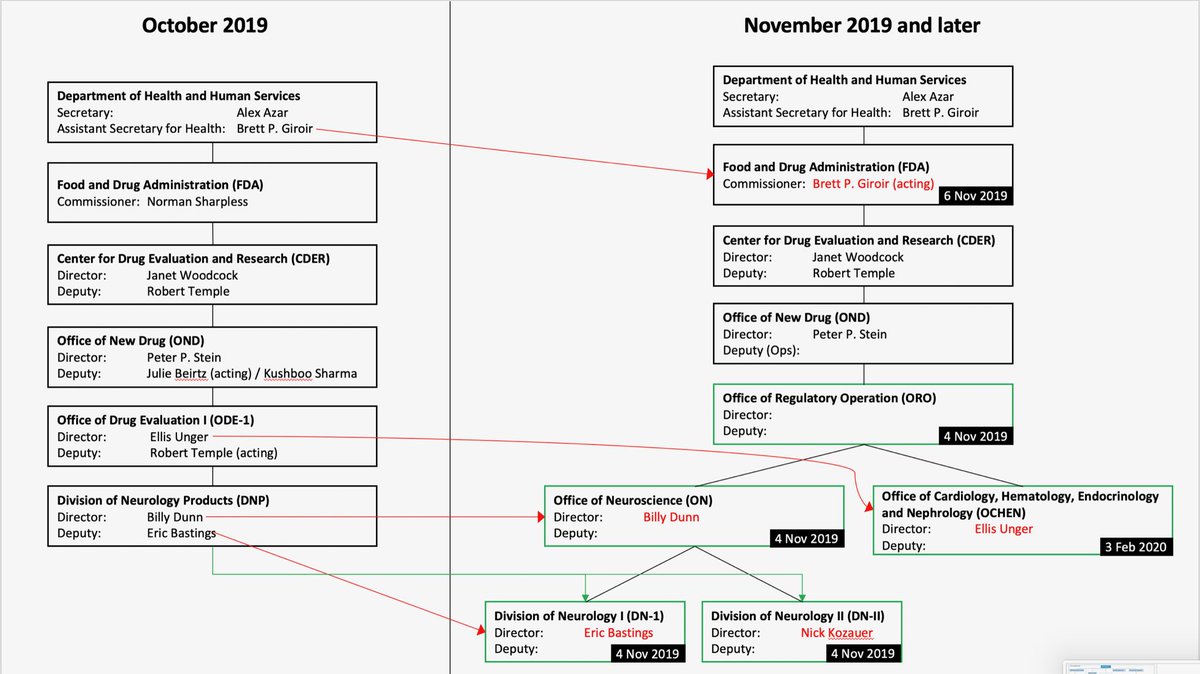
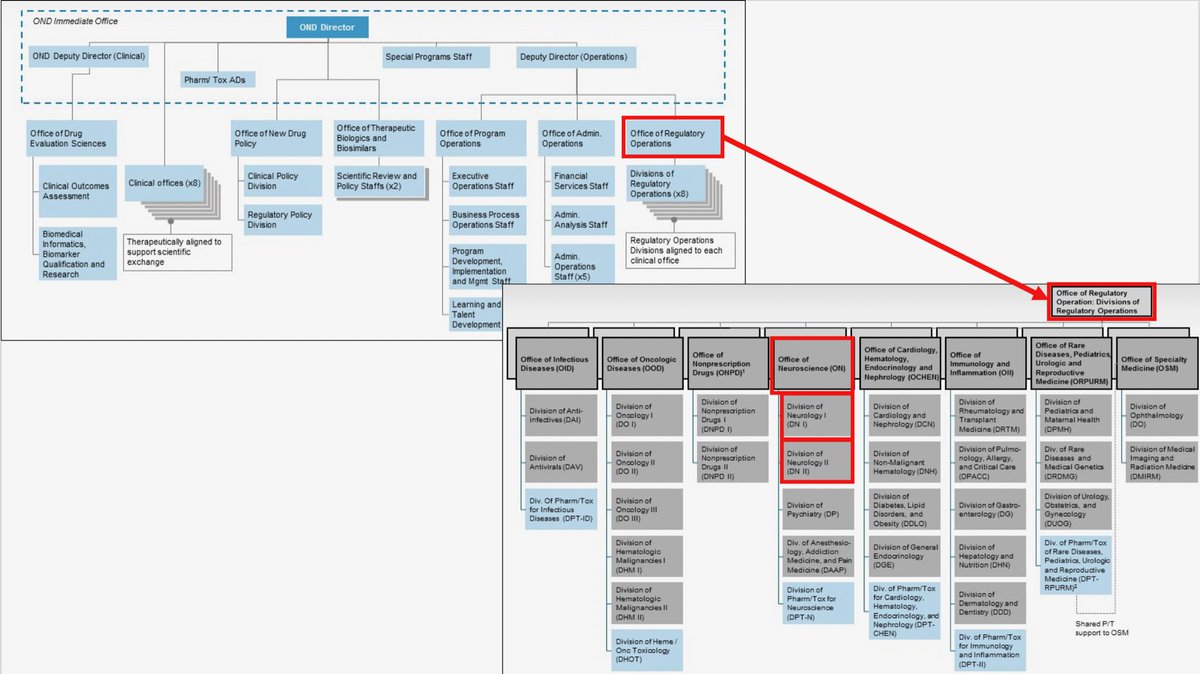
Reorganization Of The Office Of New Drugs With Corresponding .
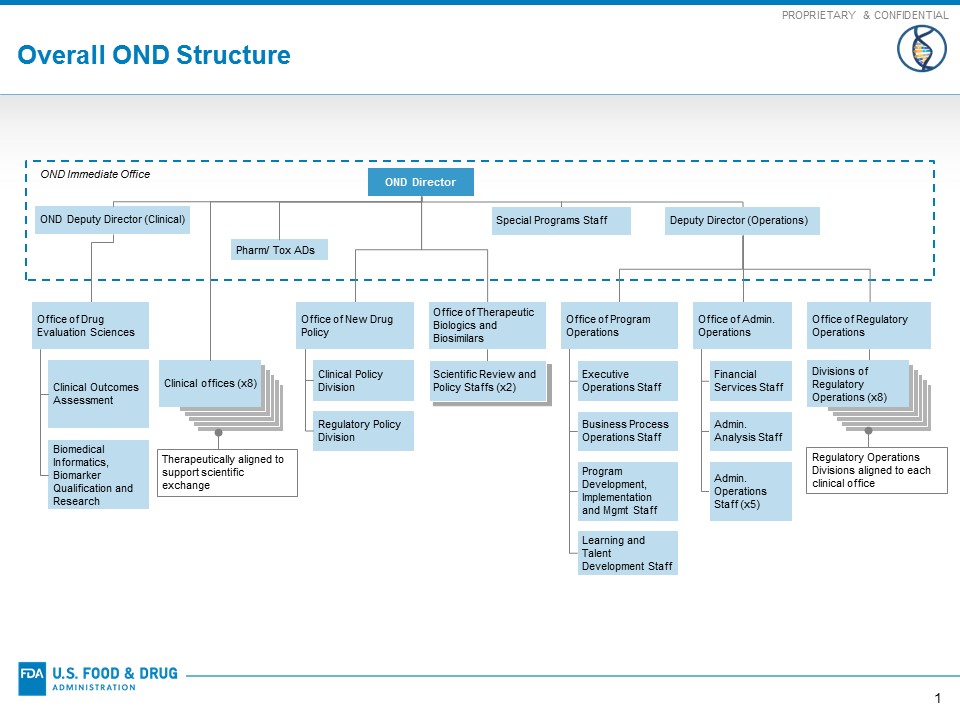
Reorganization Of The Office Of New Drugs With Corresponding .

Center For Drug Evaluation And Research Cder Tanya Eberle .

Office Of Biostatistics Center Of Drug Evaluation And .

1 Office Of Biostatistics Center Of Drug Evaluation And .
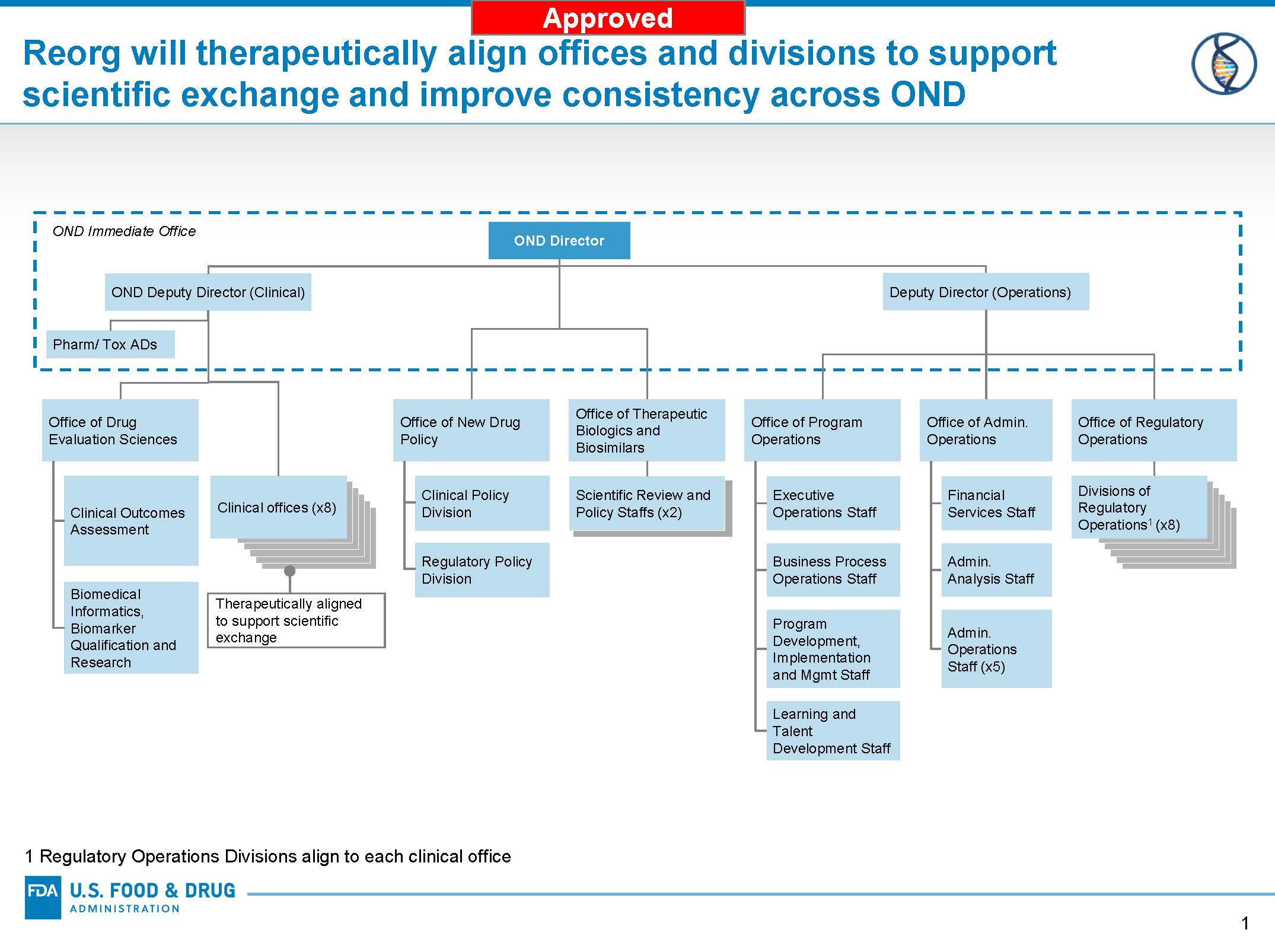
Reorganization Of The Office Of New Drugs With Corresponding .

Cder Compliance Super Office Structure Supply Chain Threats .
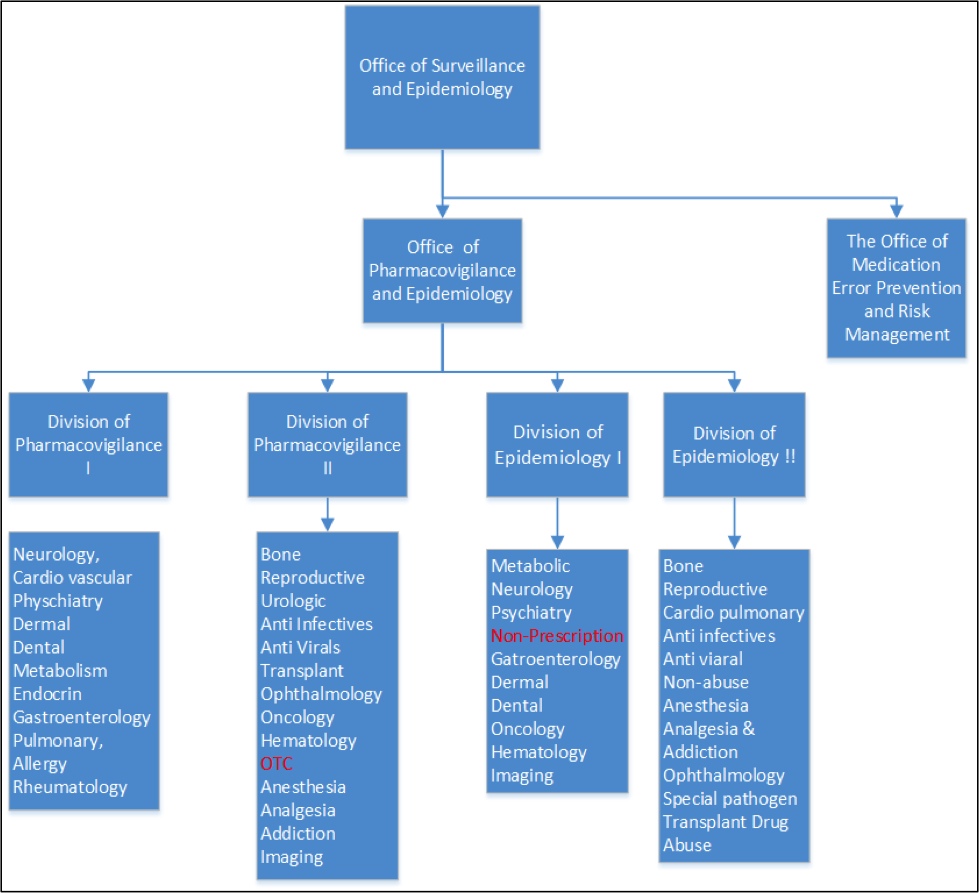
Fda Office Of Surveillance And Epidemiology The American .

21 Eye Catching Fda Cber Org Chart .

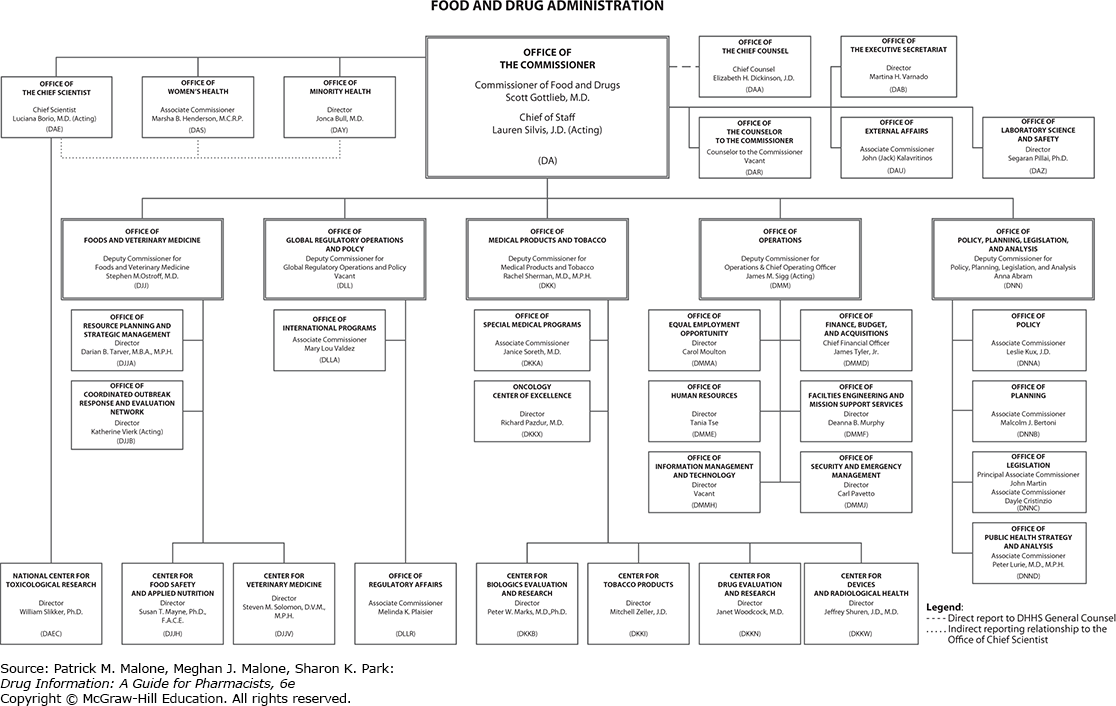
Organizational Chart .

Proposed Cder Reorganization Washington Dc Nov 4 .

Food And Drug Administration An Overview Sciencedirect .
An Overview Of The Drug Approval Process Thebodypro .
Pharmaceutical Industry And Regulatory Affairs Drug .

21 Eye Catching Fda Cber Org Chart .
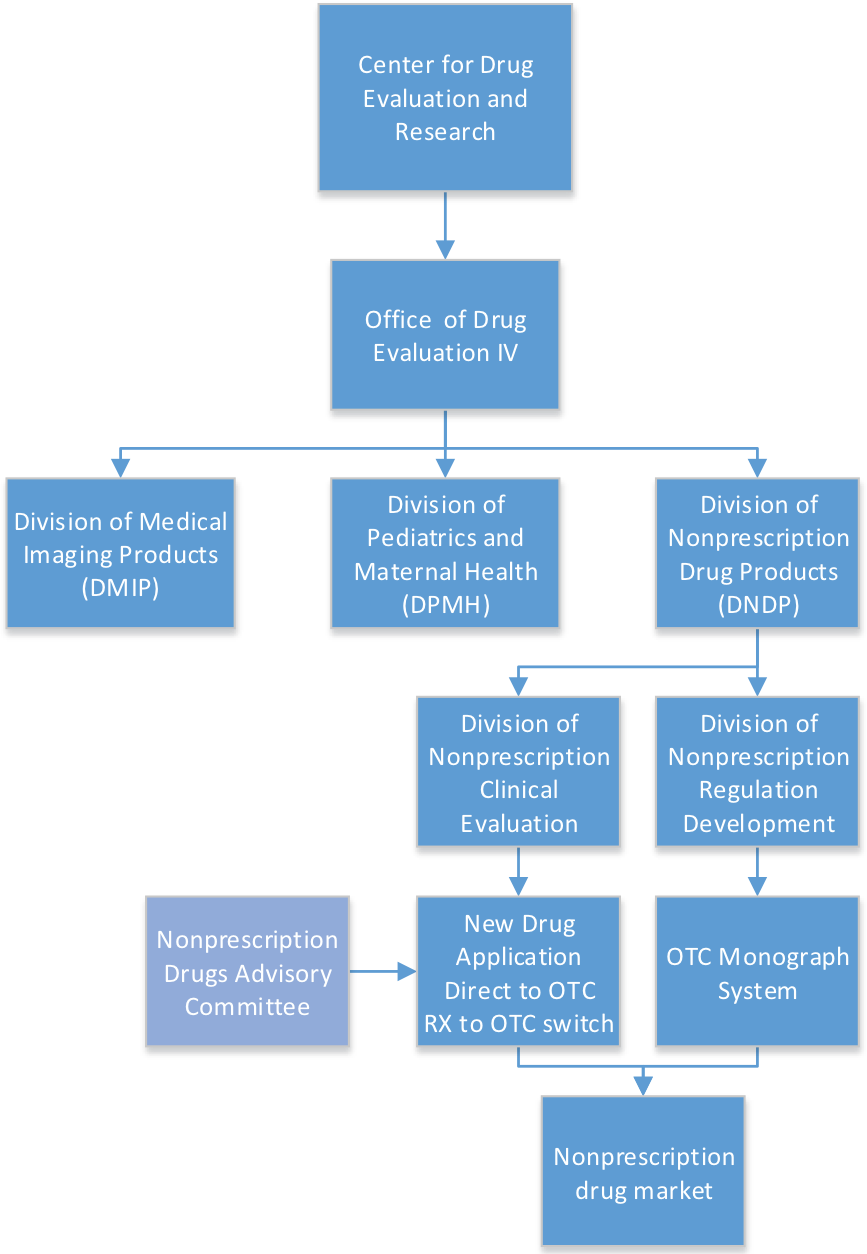
Fdas Division Of Nonprescription Drug Products The .

Center For Drug Evaluation And Research Cder .

The Us Food And Drug Administrations Center For Drug .

Title Page Quality Mo Samimi Ph D Cmq Oe Presented At The .

Working With The Fda Life Cycle Of Product Lifesciences Bc .

Figure 1 From A Program To Provide Regulatory Support For .

Meetings With Cder Judit Milstein Pdf Free Download .
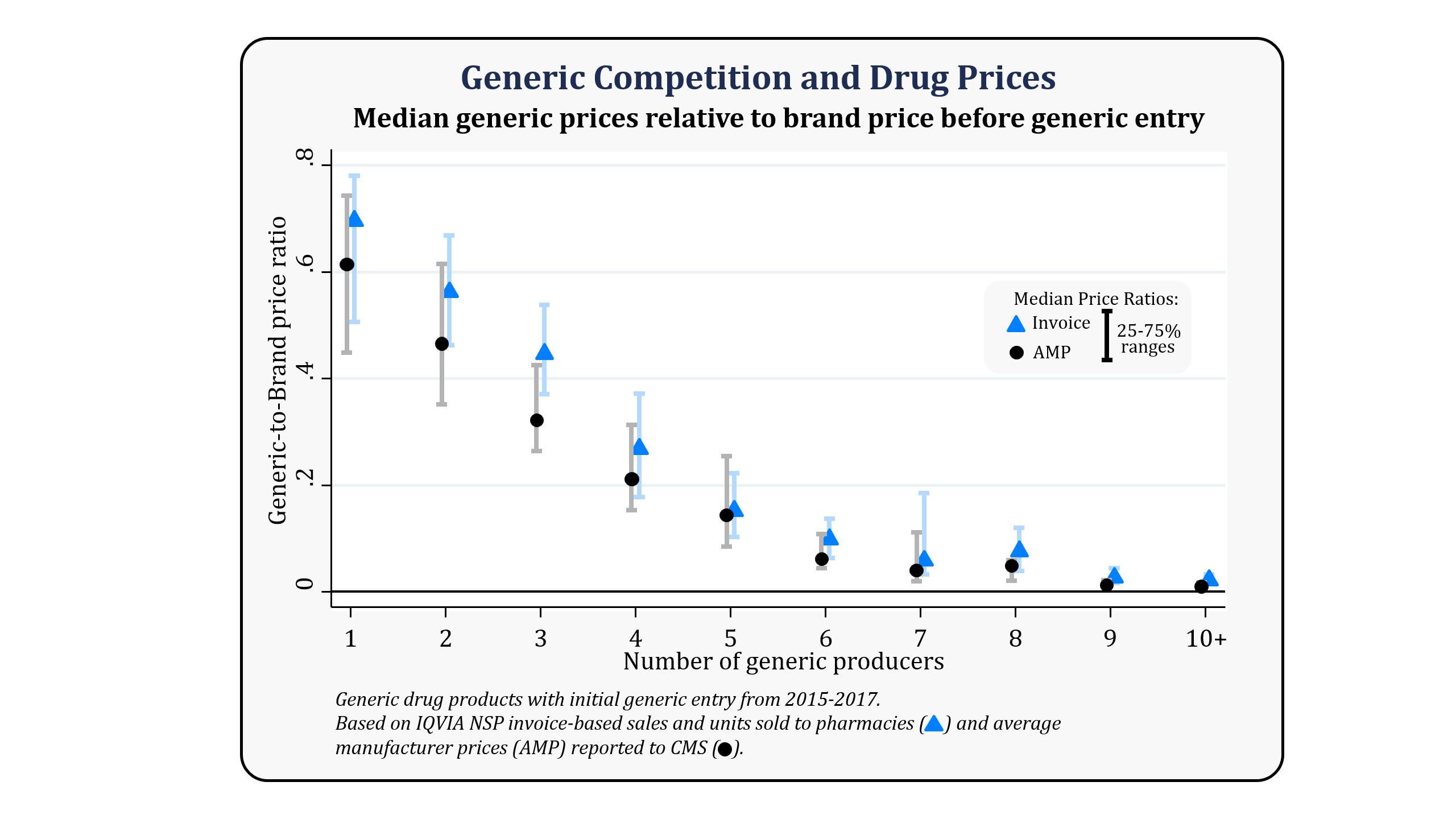
Generic Competition And Drug Prices Fda .

Ascrs Advocacy Prompts Fda To Propose Separate Drug Division .

Sidelining Safety The Fdas Inadequate Response To The Iom .

Fda Pai Compliance Program Guidance Cpg 7346 832 Ipq .

Figure 2 From Clinical Trials In Orthopaedics Research Part .
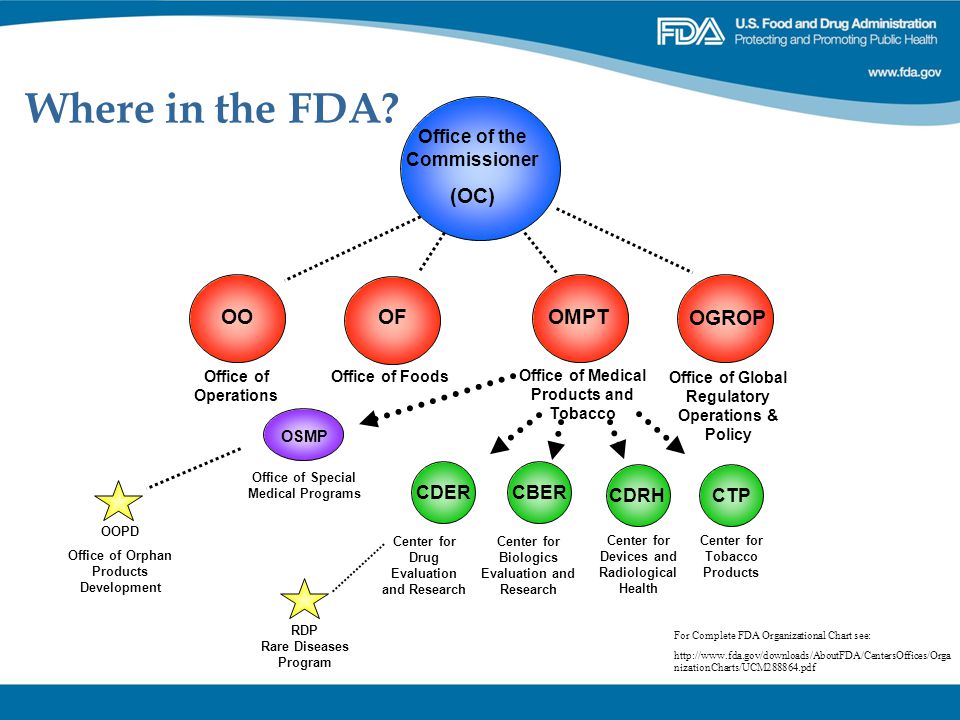
Rare Diseases And Fdasia Ppt Video Online Download .
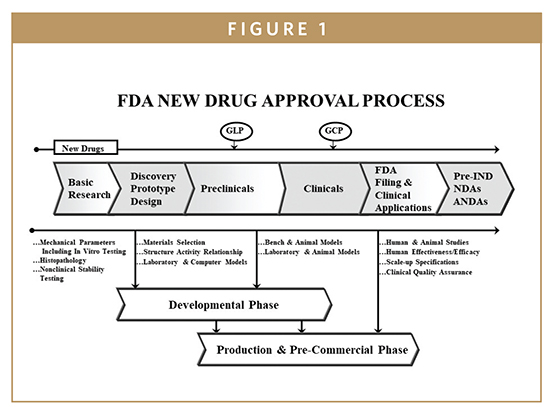
Fda Update The Fdas New Drug Approval Process .

Summary Of Fda Regulations On Exemption From Ind Research .

Fda Module 1 Grouped Submissions Q A The Ectd Summit .
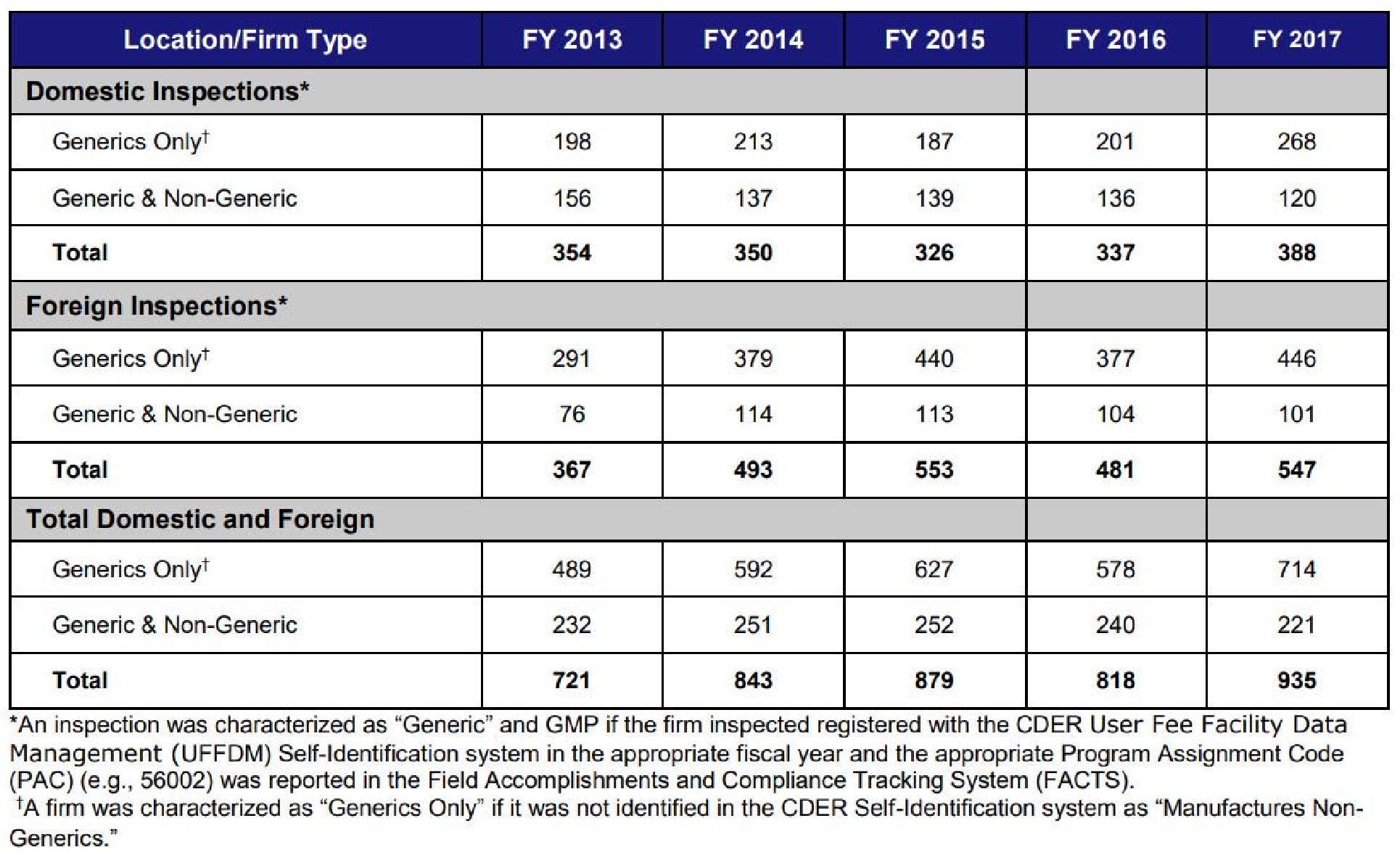
Generic Prescription Drugs Your Health Our Commitment .

Summary Level Information And Data For Cder S Inspection .

Office Of Biostatistics Center Of Drug Evaluation And .

Meetings With Cder Judit Milstein Pdf Free Download .

Product Registration And Drug Approval Process In The United .

Data Driven How Oncologists Perceive Fda Approved Drug .

Novel Fda Drug Approvals For 2017 Sharevault .

Center For Drug Evaluation And Research Cder .

Novel Fda Drug Approvals For 2017 Sharevault .

Summary Of Fda Regulations On Exemption From Ind Research .

Summary Level Information And Data For Cder S Inspection .

Drug Product Approval In The United States And International .
Pharmaceutical Industry And Regulatory Affairs Drug .
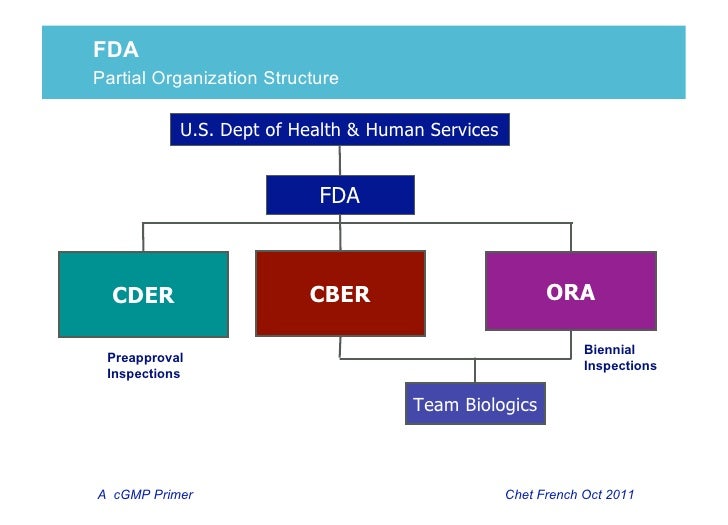
Fda Cder Organizational Chart 2018 Related Keywords .
- gram weight conversion chart
- table chart 11 to 20
- united states chart
- sample medical chart pdf
- merengue charts
- pie chart animal testing
- powerball number chart
- crocs size chart m11
- asics shoe size chart compared to nike
- ps4 selling chart
- 50 day squat challenge chart
- using vba to create charts in excel
- baby chick growth chart
- jira gantt chart plugin by frank polscheit
- square foot gardening planting chart pdf
- toledo zoo amphitheater seating chart
- kalyan guessing chart
- guitar chord substitution chart
- cheap flight chart
- abc assessment chart
- comparison chart
- ubisoft stock price chart
- scorpion savannah pants size chart
- today chart
- reem acra size chart
- pump wear ring clearance chart
- fruits ingredients chart
- what do flow charts look like
- monero cryptocurrency chart
- excel chart bring series to front